
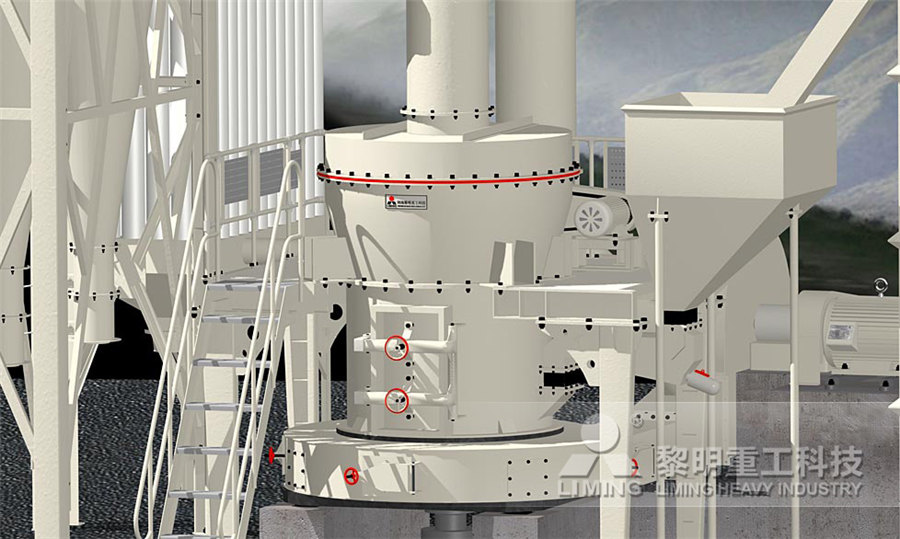
What is the machine called for the powdered calcium carbonate head
.jpg)
Calcium Carbonate Manufacturing Process and Equipment
Calcium carbonate (CaCO3) comprises more than 4% of the earth’s crust and is found worldwide Its most common natural forms are chalk, limestone, and marble (produced by the sedimentation of small fossilized shellfish, snails, and coral over millions of years) Chalk 展开2021年7月22日 In order to protect the environment, auxiliary calcium carbonate processing and dust removal equipment can be equipped Calcium carbonate jaw crusher The grinding What equipment does the calcium carbonate powder Calcium Carbonate (GCC) produced from chalk, limestone, calcite or marble have developed in recent years from just being a simple cheap filler to highest quality functional additives GCC is used as dry powder or slurry in many industrial Calcium Carbonate (GCC) Hosokawa Alpine2020年6月23日 The production of calcium carbonate fillers (GCC) from chalk, limestone or marble is challenging today GCC stands for dry powder or a suspension and is indispensable CALCIUM CARBONATE (GCC) Hosokawa Alpine

Analyzing Particle Size of Calcium Carbonates AZoM
Calcium carbonates are available with varied surface coatings and particle sizes in order to provide this range of functionality The Mastersizer 3000 laser diffraction system is capable of 2000年12月4日 Powdered calcium carbonate is produced by two methods on the industrial scale It is quarried and ground from naturally occurring deposits and in some cases Calcium Carbonate Carr Major Reference Works Wiley 2012年1月1日 The calcium carbonate was characterized by Xray powder diffraction device (XRD) and scanning electron microscopy (SEM) The optimum preparation conditions were Preparation of Calcium Carbonate Superfine Powder by Calcium 2021年11月1日 Most reported research uses Calcium Chloride (CaCl 2) as a salt precursor as a source of calcium ions [25] and Sodium carbonate (Na 2 CO 3) or potassium carbonate K 2 Structure and phase analysis of calcium carbonate powder
.jpg)
The overview of reactors used for the production
2019年5月9日 Carbonation of the Ca(OH)(2) slurry is an important method for the industrial manufacture of precipitated calcium carbonate (PCC), wherein the hydroxide particles are gradually converted toSpacefilling model of part of the crystal structure of calcium carbonate, CaCO₃ [Wikimedia] CaCO₃ is a widespread compound found in chalk, lime, marble, and more This substance is a crucial pillar of human life – it is used in Calcium carbonate, hydrochloric acid, and their Types of calcium carbonate: Consider the types of calcium carbonate offered by the manufacturer Calcium carbonate is available in different forms, such as calcium carbonate with vitamin D, calcium carbonate with magnesium, and What is calcium carbonate? formula and uses2017年1月1日 Calcium carbonate (CaCO3) is the most widely used filler material in paper, paint, plastic, food, ceramic, cosmetic, medicine and other industries In the present paper, precipitated calcium Precipitated Calcium carbonate production,
.jpg)
Calcium Carbonate(CaCO3) Limestone Formula, Structure,
2023年12月26日 Calcium Carbonate is commonly known as limestone or chalk It is often used in construction materials, like cement and mortar In our bodies, it plays a role in forming bones and teeth You can also find it in dietary supplements as a source of calcium Calcium Carbonate Formula Calcium carbonate has the chemical formula CaCO 32024年5月21日 Calcium carbonate is an important chemical compound made up of one atom of calcium bonded to one atom of carbon and three atoms of oxygenIts molecular formula is CaCO mon names for this compound include limestone, calcite, aragonite, chalk, and marble, and while all contain the same substance, each has different processes underlying its formationWhat is Calcium Carbonate? (with pictures) AllTheScience2024年7月11日 Structure of Calcium Carbonate Calcium carbonate is characterized by its unique chemical structure, represented by the formula CaCO₃At its core, this compound consists of one calcium (Ca) ion bonded to one carbonate (CO₃) ion The carbonate ion is a polyatomic ion with a trigonal planar geometry, comprising one carbon atom centrally bonded to three oxygen Calcium Carbonate(CaCo₃) Definition, Structure, Properties, 2017年7月9日 The reaction routes for ex situ mineral carbonation can be divided into two processes—direct and indirect mineral carbonations A direct carbonation is the simplest carbonation method, where Ca or Mg feedstock directly reacts with CO 2 in a single step, and further it can be conducted by gas–solid or aqueous route (Eloneva et al, 2007)Direct Frontiers Calcium Carbonate Precipitation for CO2 Storage
Calcium carbonate Uses, Side Effects Warnings Drugs
2023年8月1日 What is calcium carbonate? Calcium is a mineral that is found naturally in foods Calcium is necessary for many normal functions of the body, especially bone formation and maintenance Calcium carbonate is used to prevent or to treat a calcium deficiency There are many brands and forms of calcium carbonate available2013年5月20日 2 Some powdered calcium carbonate was heated strongly in a test tube The gas given off was bubbled through limewater The equation for the reaction taking place in the heated tube is Heptane belongs to a homologous series of compounds called alkanes The general formula of the alkanes is C n HEdexcel International GCSE Chemistry Pearson Calcium Carbonate Formula It is a chemical compound with the chemical formula CaCO 3; It is a white insoluble powderlike substance which occurs naturally in minerals, chalk, marble, limestone, calcite, shells, pearl, etc; Medicinally, it is used as an antacid or as a Limestone: Calcium Carbonate (CaCO3) Uses, Preparation, Balanced chemical equation of CaCO 3 and HCl reaction with physical states CaCO 3(s) + 2HCl (aq) → CaCl 2(aq) + CO 2(g) + H 2 O (l) Calcium carbonate is not soluble in water and exists as white precipitate in the water When aqueous hydrochloric acid is added, you can observe air bubbles are generated and calcium chloride, carbon dioxide and water are formedCalcium Carbonate and Hydrochloric Acid Reaction CaCO
.jpg)
(PDF) Biogenic Fishgut Calcium Carbonate is a
2013年4月23日 Fish excrete carbonate at high rates (ie, at least up to 105 g m −2 yr −1 on coral reefs) 28 The global significance of this process lies in the typically high Mg/Ca ratios and low degrees Huber manufactures calcium carbonate granulations using our own highpurity powder at our manufacturing facility in Quincy, Illinois Exceptional Value vs Precipitated Calcium Carbonate With HuberCal calcium carbonate powder, Calcium Carbonate Powder HuberCal Calcium 2021年10月15日 In the study, FTIR spectra for calcium carbonate appeared at 8641 and 71114 cm −1 bands and at 147362 and 139222 cm −1 bands, which correspond to respective CaO and CO bonds, matching with a high concentration of CaCO 3 as observed in the samples The FTIR results can also characterize the types of crystals formed based on the The potential use of green mussel (Perna Viridis) shells for Other calcium carbonate brands include: Alcalak, AlkaSeltzer Cool Action Heartburn Relief Gum, AlkaSeltzer Heartburn ReliefChews, Alkets, Alkums, AmiLac, Amitone, Cal Oys, CalGest, CalMint, Calcarb, Calci Mix, CalciChew, Calcid, Calcitab, Calcium Concentrate, Calcium Liquid Softgel, Calcium Oyster, Calcium Oyster Shell, Caltrate, Caltro Comparing Calcium Carbonate vs Tums Regular Strength
.jpg)
78: Acid–Base and Gas Evolution Reactions Chemistry
The reaction of acid and base to make water and a salt is called neutralization Like any chemical equation, a neutralization chemical equation must be properly balanced For example, the neutralization reaction between sodium hydroxide and hydrochloric acid is as follows: Sulfuric acid reacts with calcium carbonate to form calcium sulfate Calcium carbonate is one of the most useful and versatile materials known to man This family of essential minerals comprises more than four percent of the earth’s crust and is found worldwide It is produced by the sedimentation of the shells of small fossilized snails, shellfish, and coral over millions of years The most common forms of calcium carbonate are chalk, limestone, and Calcium Carbonate Essential Minerals AssociationWhat Is Calcium Carbonate Four Uses of Calcium Carbonate Calcium Carbonate Production Process Ground calcium carbonate is also called known as heavy calcium carbonate,it is made by mechanical methods (using grinding mills such as roller mill or other highpressure grinding mills) to directly crush natural calcite, limestone, chalk, shells, etc Ground calcium carbonate Calcium Carbonate – What Is It And How To Make ItCalcium carbonate, CaCO 3 (s) – see CLEAPSS Hazcard HC019b The calcium carbonate used should be in the form of pea sized lumps of chalk Blackboard chalk should not be used as it is likely to be mostly calcium sulfate Universal indicator solution (HIGHLY FLAMMABLE) – see CLEAPSS Hazcard HC032 and CLEAPSS Recipe Book RB047 ProcedureThermal decomposition of calcium carbonate RSC Education

Ground Calcium Carbonate / Limestone Hosokawa Micron Powder Systems
Pure calcium carbonate is a relatively soft mineral/compound, with a Mohs hardness of 25 – 30 However, mined limestone may have a minor portion of abrasive impurities such as quartz / sand One of the standard methods of milling Limestone/Calcium Carbonate is with the Mikro ACM Air Classifying Mill The ACM has a rotating rotor disc with 2019年1月23日 7 Repeat Steps 1–6 with 20 g of small calcium carbonate chips and 20 g of powdered calcium carbonate Part B: Concentration Note: The small chips with 20 M HCl have already been measured in Part A; just transfer the data to the new results table 1 Repeat Part A with small chips only and changing the concentration of HCl to 10 M and 05 MExperiment 191: Investigating the rate of chemical 1 The calcium supplements taken by many women are composed primarily of powdered calcium carbonate, CaCO3, which is also the primary component of marble a) Briefly explain why CaCO3 would be a good source for a woman suffering from chronic heart burn, use a net ionic equation, b) Marble statues erode when exposed to acidic precipitationSolved 1 The calcium supplements taken by many women are Chegg2023年8月5日 Calcium carbonate is an inorganic salt primarily used to manage and treat low calcium conditions, GERD, CKD, and other indicated conditions Calcium carbonate is classified as a calcium supplement, antacid, and phosphate binder This activity outlines the significant indications, actions, and contraindications for calcium carbonate as a valuable agent in Calcium Carbonate StatPearls NCBI Bookshelf

Calcium carbonate Formula, Uses, Names, Facts Britannica
2024年10月26日 As marble, calcium carbonate is used for statuary and carvings and is a popular facing stone as polished slabsThe term marble is used differently in the marketplace from the way it is used in geology: in the marketplace, it is applied to any coarsegrained carbonate rock that will take a good polish rather than to metamorphic carbonaterich rocks exclusivelyFigure \(\PageIndex{1}\): (a) Stalactites and (b) stalagmites are cave formations of calcium carbonate (credit a: modification of work by Arvind Govindaraj; credit b: modification of work by the National Park Service) The two carbonates used commercially in the largest quantities are sodium carbonate and calcium carbonate186: Occurrence, Preparation, and Properties of Carbonates2021年12月28日 Calcium carbonate based filler additive materials have approximately 70% market share in North America, and can be added to a maximum of 20 to 25% based on fiber material machine by N icolas (PDF) InSitu Precipitated Calcium Carbonate Paper2023年8月25日 8 Biological Calcium Carbonate Mineralization: Calcite is essential in the formation of shells, skeletons, and other hard structures in various marine organisms, including mollusks, corals, and certain types of algae These organisms extract dissolved calcium and carbonate ions from seawater to build their protective structures 9Calcite : Properties, Formation, Occurrence and Uses Areas
.jpg)
What is calcium carbonate and what is it used for?
2022年5月25日 Calcium carbonate: the formula, properties and MSDS What is calcium carbonate? CaCO 3 is a molecular formula that defines a white or transparent mineral in the form of crystals Calcium carbonate (customary name – calcite) consists of a carbonic acid calcium salt What are the features of calcium carbonate? This inorganic compound has the following Calcium Carbonate (CaCO3) Calcium carbonate molecular formula is CaCO3 Visit BYJU'S to understand the properties, structure and Uses of calcium carbonate (CaCO3) explained by India's best teachers It can be prepared on a large scale by passing carbon dioxide gas through calcium hydroxide (otherwise called slaked lime) However, if there Calcium Carbonate (CaCO3) Structure, Properties, Uses of Calcium Calcium carbonate nanocomposites Y Lin, CM Chan, in Advances in Polymer Nanocomposites, 2012 31 Introduction: applications of calcium carbonate nanoparticles Calcium carbonate particles have been used in the plastics industry for many years The original purpose of adding ground calcium carbonate (GCC) particles as filler material for plastics was to Calcium Carbonate an overview ScienceDirect TopicsSpacefilling model of part of the crystal structure of calcium carbonate, CaCO₃ [Wikimedia] CaCO₃ is a widespread compound found in chalk, lime, marble, and more This substance is a crucial pillar of human life – it is used in Calcium carbonate, hydrochloric acid, and their

What is calcium carbonate? formula and uses
Types of calcium carbonate: Consider the types of calcium carbonate offered by the manufacturer Calcium carbonate is available in different forms, such as calcium carbonate with vitamin D, calcium carbonate with magnesium, and 2017年1月1日 Calcium carbonate (CaCO3) is the most widely used filler material in paper, paint, plastic, food, ceramic, cosmetic, medicine and other industries In the present paper, precipitated calcium Precipitated Calcium carbonate production, 2023年12月26日 Calcium Carbonate is commonly known as limestone or chalk It is often used in construction materials, like cement and mortar In our bodies, it plays a role in forming bones and teeth You can also find it in dietary supplements as a source of calcium Calcium Carbonate Formula Calcium carbonate has the chemical formula CaCO 3Calcium Carbonate(CaCO3) Limestone Formula, Structure, 2024年5月21日 Calcium carbonate is an important chemical compound made up of one atom of calcium bonded to one atom of carbon and three atoms of oxygenIts molecular formula is CaCO mon names for this compound include limestone, calcite, aragonite, chalk, and marble, and while all contain the same substance, each has different processes underlying its formationWhat is Calcium Carbonate? (with pictures) AllTheScience
.jpg)
Calcium Carbonate(CaCo₃) Definition, Structure, Properties,
2024年7月11日 Structure of Calcium Carbonate Calcium carbonate is characterized by its unique chemical structure, represented by the formula CaCO₃At its core, this compound consists of one calcium (Ca) ion bonded to one carbonate (CO₃) ion The carbonate ion is a polyatomic ion with a trigonal planar geometry, comprising one carbon atom centrally bonded to three oxygen 2017年7月9日 The reaction routes for ex situ mineral carbonation can be divided into two processes—direct and indirect mineral carbonations A direct carbonation is the simplest carbonation method, where Ca or Mg feedstock directly reacts with CO 2 in a single step, and further it can be conducted by gas–solid or aqueous route (Eloneva et al, 2007)Direct Frontiers Calcium Carbonate Precipitation for CO2 Storage 2023年8月1日 What is calcium carbonate? Calcium is a mineral that is found naturally in foods Calcium is necessary for many normal functions of the body, especially bone formation and maintenance Calcium carbonate is used to prevent or to treat a calcium deficiency There are many brands and forms of calcium carbonate availableCalcium carbonate Uses, Side Effects Warnings Drugs2013年5月20日 2 Some powdered calcium carbonate was heated strongly in a test tube The gas given off was bubbled through limewater The equation for the reaction taking place in the heated tube is Heptane belongs to a homologous series of compounds called alkanes The general formula of the alkanes is C n HEdexcel International GCSE Chemistry Pearson
.jpg)
Limestone: Calcium Carbonate (CaCO3) Uses, Preparation,
Calcium Carbonate Formula It is a chemical compound with the chemical formula CaCO 3; It is a white insoluble powderlike substance which occurs naturally in minerals, chalk, marble, limestone, calcite, shells, pearl, etc; Medicinally, it is used as an antacid or as a













